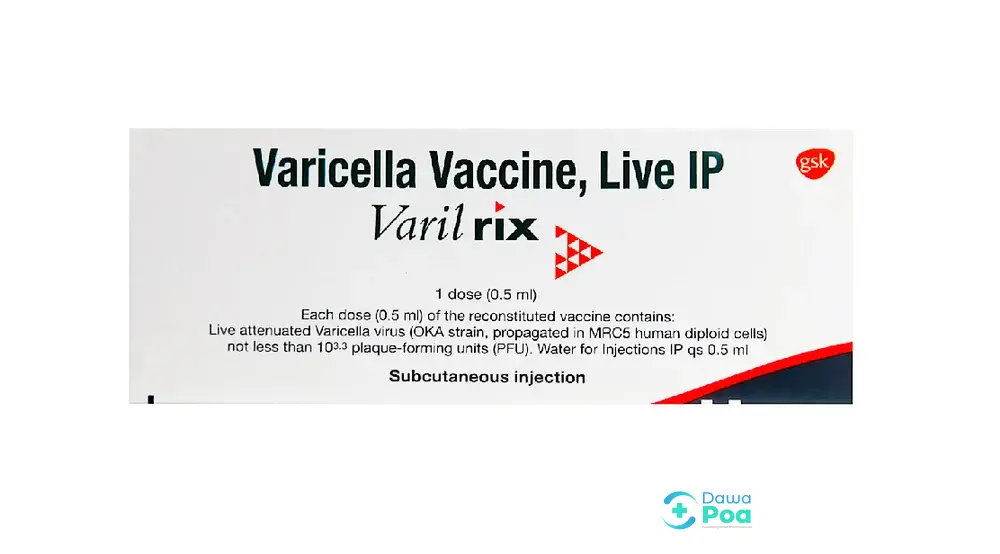
Varilrix Vial
KSh 6,999.00
Varilrix is a live attenuated lyophilized (freeze-dried) powder for injection containing the live attenuated varicella-zoster virus (Oka strain). It is used for active immunization against varicella (chickenpox). Active Substances: Each 0.5 mL dose of the reconstituted vaccine contains: Live attenuated varicella-zoster virus (Oka strain): Not less than 103.3 plaque-forming units (PFU).
Vaccine
Suggested Use
Varilrix is indicated for:
- Active immunization against varicella in healthy individuals aged 12 months and older.
- Post-exposure prophylaxis for healthy, susceptible individuals exposed to varicella within 72 hours of contact.
- Individuals at high risk of severe varicella.
Dosage:
- Children (12 months to 12 years): Two doses, with the second dose administered at least 3 months after the first.
- Adolescents and Adults: Two doses, with a minimum interval of 4 weeks between doses.
- Administration: The vaccine should be injected subcutaneously or intramuscularly.
Warning
- Varilrix is contraindicated in individuals with severe immunodeficiencies or those who are pregnant.
- Caution should be exercised in individuals with bleeding disorders; subcutaneous administration is recommended for these patients.
- Common side effects may include mild pain, swelling, and redness at the injection site, fever, and rash. These are typically temporary but should be monitored closely.
Storage and Handling
- Store Varilrix at temperatures between 2 °C and 8 °C, protected from light.
- The powder must be reconstituted with the provided solvent before use, and it should be shaken well after reconstitution.
Always consult a healthcare professional for personalized advice and to ensure safe administration of the vaccine.