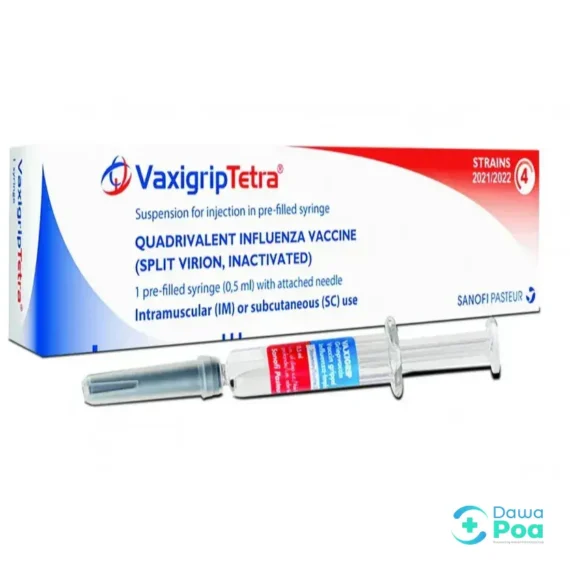
Vaxigrip Tetra Inj 1’s
KSh 1,915.00
Vaxigrip Tetra is a quadrivalent inactivated influenza vaccine meant to provide active immunization against influenza types A and B viruses. It is suitable for adults and children aged 6 months and older. Vaxigrip Tetra is an inactivated influenza vaccine designed to protect against four different strains of the flu virus.
Vaccine
Suggested Use
Vaxigrip Tetra is indicated for the prevention of influenza disease caused by the influenza virus subtypes and influenza B virus types contained in the vaccine. It is suitable for:
- Active immunization of adults, including pregnant women, and children from 6 months of age and older.
- Passive protection of infants from birth to less than 6 months of age following vaccination of pregnant women.
Dosage:
- Adults and children over 36 months of age: One dose of 0.5 mL.
- Children from 6 to 35 months of age: Clinical data are limited. Doses of 0.25 mL or 0.5 mL have been used.
- Infants less than 6 months of age: The safety and efficacy of Vaxigrip Tetra administration have not been established.
- Children less than 9 years of age who have not previously been vaccinated: A second dose of 0.5 mL should be given after an interval of at least 4 weeks.
Warning
- Contraindicated in individuals with known hypersensitivity to any component of the vaccine, including egg proteins.
- Vaccination should be postponed in cases of moderate or severe febrile illness.
- The vaccine may not provide complete protection in all individuals, especially those with compromised immunity.
- Immediate medical attention should be available in case of anaphylactic reactions.
- Safe for use during pregnancy and breastfeeding; consult a healthcare provider for personalized advice.
- Store between 2 °C and 8 °C. Do not freeze.
Side Effects:
- Common: Soreness, redness, or swelling at the injection site, mild fever, muscle aches, and fatigue.
- Rare: Severe allergic reactions, Guillain-Barré syndrome, or other neurological complications.
Always consult a healthcare provider for advice on vaccination suitability and management of side effects.